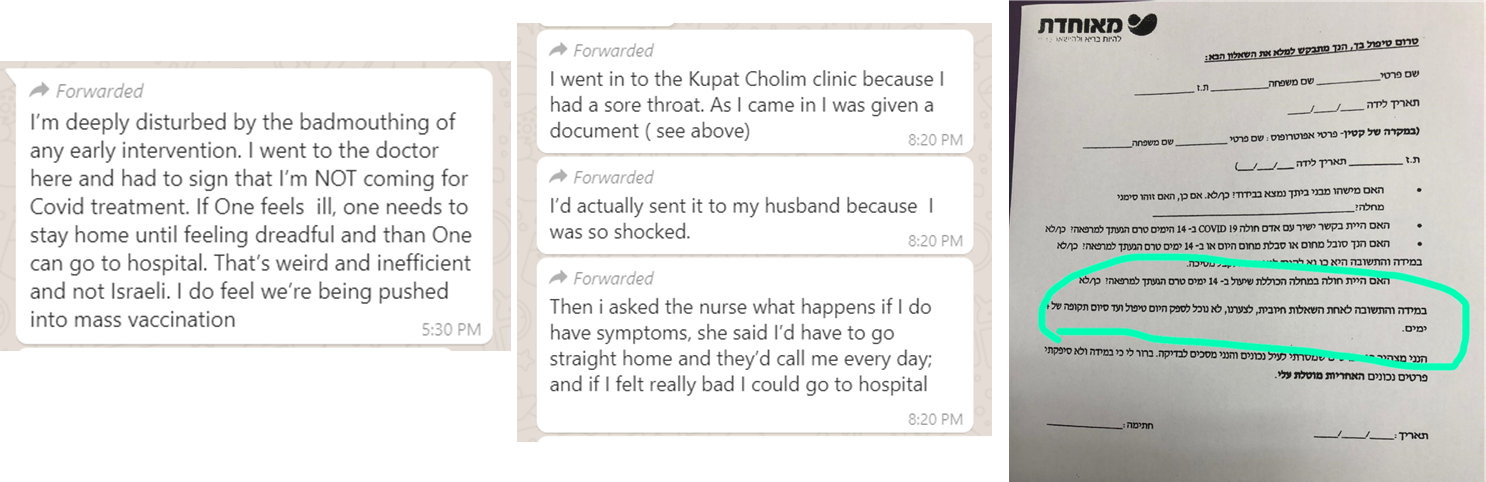
WhatsApp message – July 13, 2020
The document (to the right) which the writer refers to is a questionnaire from her HMO in Israel, referred to as the kupat cholim. The patient is asked about their symptoms and contact with anyone diagnosed positive with Covid-19. Any affirmative answer, including fever or cough within the last 14 days means that the patient would not be seen in the clinic at all, merely monitored over the phone, and could not come back to the clinic for 14 days. Only if and when symptoms would worsen significantly would the patient be directed to go to the emergency room.
The early intervention the writer is referring to is the drug hydroxychloroquine.
Formerly titled “About Medical Experts and Consensus in Light of the Hydroxychloroquine Debacle”
Table of Contents
Hydroxychloroquine – yay or nay for treating Covid-19?
Great controversy surrounds the use of Hydroxychloroquine to treat Covid-19 patients. This is a drug that’s been used for over 65 years and known to be safe and effective. Why should there be any controversy about? If there are so many doctors who have extolled its effectiveness, why are other doctors suddenly against it? Some national and local governments have banned it; the FDA, WHO, and the CDC will not recommend its use as treatment for Covid-19; and some pharmacists will not fill prescriptions for it. Other national and local governments promote its use, some doctors swear by it, and patients claim that it’s cured them. How could there suddenly be such different views about a drug with such a reliable history? Why are doctors and media quickly removed and deplatformed by Twitter, Facebook, and YouTube, for promoting hydroxychloroquine to treat Covid-19, labeling it misinformation and fake news.
How can we tell where and with whom the truth lies?
What is hydroxychloroquine?
Hydroxychloroquine (HCQ) is a 65 year old malaria drug which has also been used to treat patients with rheumatoid arthritis, lupus, and autoimmune diseases. It is on the WHO List of Essential Medications. It has a long history of safe and effective use1 and is the 128th most prescribed drug in the United States with 5.6 million prescriptions filled in 20172.
Hydroxychlorquine provides several valuable functions to help your body fight this virus, the most discussed being its ability to help zinc enter the cells because zinc works to eliminate the virus. It is therefore recommended to prescribe zinc with the HCQ. An antibiotic such as azythromycin (Zythromax or Z-pac) is often prescribed together with the HCQ in order to prevent secondary bacterial infections such as pneumonia. Doctors have found this treatment protocol to be most effective in patients during the early stage of the illness when the amount of virus is limited and is, therefore, easier to eliminate.
Hydroxychloroquine, zinc, and azythromyacin are inexpensive drugs, readily available, and easily affordable. Not only is this a safe and affordable treatment for Covid-19, but it had already been established as an effective remedy for a similar coronavirus.
Hydroxychlorquine studied as remedy for SARS
SARS was a coronavirus that appeared in 2002. While it had a higher 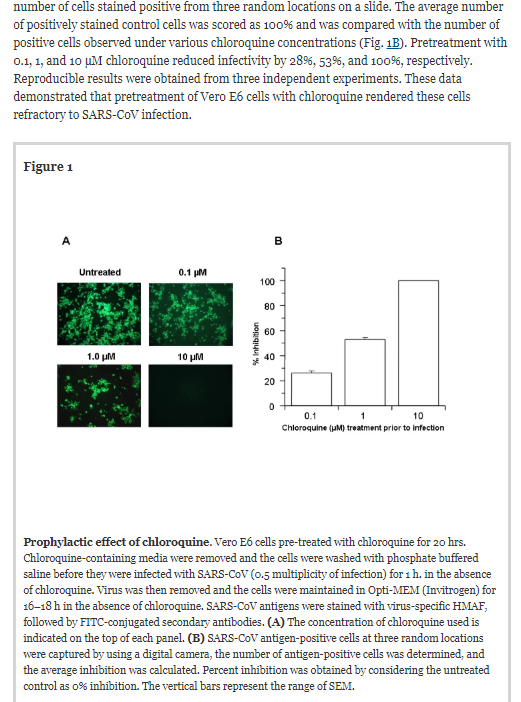 fatality rate than Covid, it was not as contagious. In an attempt to find a cure for SARS, in-vitro studies using primate (monkey) cells showed that chloroquine and hydroxychloroquine (a safer form) were effective against the coronavirus. Five of the study’s eight authors worked at the CDC and three worked at the Clinical Research Institute of Montreal. They found that it had possible preventive and therapeutic benefits:
fatality rate than Covid, it was not as contagious. In an attempt to find a cure for SARS, in-vitro studies using primate (monkey) cells showed that chloroquine and hydroxychloroquine (a safer form) were effective against the coronavirus. Five of the study’s eight authors worked at the CDC and three worked at the Clinical Research Institute of Montreal. They found that it had possible preventive and therapeutic benefits:
Chloroquine, a relatively safe, effective and cheap drug used for treating many human diseases including malaria, amoebiosis and human immunodeficiency virus is effective in inhibiting the infection and spread of SARS CoV in cell culture. The fact that the drug has significant inhibitory antiviral effect when the susceptible cells were treated either prior to or after infection suggests a possible prophylactic and therapeutic use3.
Chloroquine was identified in 1934. Its antiviral capabilities must have been well known for quite some time; on the heels of the SARS epidemic, a 2003 episode of the TV series Dead Zone featured a newly discovered and deadly virus. Chloroquine was identified as the cure for the virus and the patients who received it all recuperated4.
Anthony Fauci, head of the US Coronavirus Task Force, has been the director of the National Institute of Allergy and Infectious Diseases (NIAID), an arm of the NIH, since 1984. As director of the NIAID, Fauci oversees an extensive portfolio of basic and applied research to prevent, diagnose, and treat established infectious diseases such as HIV/AIDS, respiratory infections, diarrheal diseases, tuberculosis and malaria as well as emerging diseases such as Ebola and Zika. NIAID also supports research on transplantation and immune-related illnesses, including autoimmune disorders, asthma and allergies5. He should have known about the benefit of hydroxychloroquine6.
Why didn’t Fauci suggest using hydroxychloroquine to mitigate the effects of the pandemic, especially after other recent international studies and doctors’experience with it proved positive?
Use of hydroxychloroquine at early stage found to be effective against Covid-19
Nationally and internationally, numerous trials of hydroxychloroquine have repeatedly proven its effectiveness, even at the beginning of the pandemic. Yet, inexplicably, these studies are denigrated or ignored by the WHO, FDA, and other health agencies that state that there is no cure for this coronavirus. What could be their reason for denying patients a life saving treatment?
Here are a few of the studies and doctors’ experiences.
♦ China and South Korea find HCQ effective against Covid-19
A March 13, 2020, white paper presented by Dr. James M. Todaro, MD and Gregory J. Rigano, Esq. in consultation with Stanford University School of Medicine, UAB School of Medicine and National Academy of Sciences researchers discussed three studies where HCQ was found effective in treating Covid-19 – the 2005 US study and clinical studies in China and South Korea, two countries that encountered the virus well before the US did. SARS and Covid-19 share most of the same genome7.
♦ Eminent French doctor finds HCQ and azythromycin effective against Covid-19
Prof. Dideur Raoult, a leading French infectious disease specialist, who founded and directs the Institut Hospitalo-Universitaire Méditerranée Infection (IHU), a research hospital, declared that Covid-19 is easy to treat with hydroxychloroquine and azythromyacin.
Raoult decided to trial hydroxychloroquine based upon the study from China showing effectiveness against Covid-19. This was not his first experience with HCQ, however. As he explained:
Our team has a very comprehensive experience in successfully treating patients with chronic diseases due to intracellular bacteria (Q fever due to Coxiella burnetii and Whipple’s disease due to Tropheryma whipplei) with long-term hydroxychloroquine treatment (600 mg/day for 12 to 18 months) since more than 20 years. [15,16] We therefore started to conduct a clinical trial aiming at assessing the effect of hydroxychloroquine on SARS-CoV-2-infected patients after approval by the French Ministry of Health8.
Raoult conducted two clinical trials and a third, larger, retrospective study. All three showed the benefit of hydroxychloroquine and azythromycin when patients were treated early in the illness. The third study showed a cure rate of about 98%9’10’11’12.
Why has Raoult, an established expert in his field, been besmirched and threatened for promoting hydroxychloroquine?
♦ Survey of doctors treating Covid-19 patients
Sermo, a social media platform for doctors, issued an extensive and detailed report of their March 25-27, 2020 survey of 6,227 physicians across all specialties regarding Covid-19. Doctors from 30 countries were surveyed; countries included were the United States, Canada, Argentina, Brazil, Mexico, Germany, Italy, UK, France, Spain, Belgium, Netherlands, Sweden, Turkey, Poland, Russia, Finland, Ireland, Switzerland, Austria, Denmark, Norway, Greece, Taiwan, Japan, South Korea, Australia, China, India, and Hong Kong13. They found that:
Hydroxychloroquine usage amongst COVID-19 treaters is 72% in Spain, 49% in Italy, 41% in Brazil, 39% in Mexico, 28% in France, 23% in US, 17% in Germany, 16% in Canada, 13% in UK and 7% in Japan.
- Hydroxychloroquine was overall chosen as the most effective therapy from a list of 15 options (37% of COVID-19 treaters)14.
Azythromycin or similar antibiotics were reported to have the next highest usage at 32% for Covid-19 treaters overall15.
♦ NY physician Dr. Vladimir (Zev) Zelenko develops a successful HCQ protocol
Dr. Vladimir Zelenko, a NY doctor serving about 75% of the adults in a community of 35,000 for the past 16 years, began a HCQ, azythromycin, and zinc protocol for his patients during the early stages of illness. He developed his protocol based upon the data from the Chinese, South Korean, and French study reports. Of the 500 patients that he and his team had treated by March 23, 2020, none of his patients died, nor where they hospitalized or intubated. Only 10% reported mild side affects such as temporary nausea or diarrhea 16. By April 5, 2020, he treated 900 patients with a 99.9% success rate, success defined as no deaths17
♦ Henry Ford Health System Study
On July 1, 2020, the Henry Ford Health System, a large six hospital integrated health system in Michigan, the largest of the hospitals being an 802-bed quaternary academic teaching hospital in Detroit, published the results of their retrospective study for the period March 10, 2020 to May 2, 2020. They studied three groups: patients who only received hydroxychloroquine, who received hydroxychloroquine in combination with azithromycin, azithromycin alone, or who received neither of the medications. Patients treated with HCQ only benefited about 66% and those treated with both hydroxychloroquine + azithromycin had a 71% benefit as compared to those treated with neither drug18.
♦ America’s Frontline Doctors
On July 27, 2020, several doctors gathered in front of the US Supreme Court to tell the public not to worry about Covid-19 and that, among other things, hydroxychloriquine along with zythromax and zinc was effective against Covid-19 as a prophylactic and in the early stages of the illness. This press conference was followed by a two session summit to empower people not to live in fear; session two discussed the biomechanics of HCQ – how it works at the cellular level19’20
♦ Dr. Steven Hatfill
Virologist Steven Hatfill blasted the media for their continuous denigration of hydroxychloroquine, an effective treatment for Covid-1921
♦ Dr. Harvey Rich
Yale epidemiologist, Dr. Harvey Risch, has accused Dr. Fauci of waging a misinformation campaign about hydroxychloroquine stating that it has proven effective against Covid-19 for many patients 22.
These doctors are established experts and/or have successfully treated many covid patients with HCQ. Why have they been ridiculed in the media and even deplatformed on social media for disseminating important information when it could help save lives?
♦ A natural experiment confirms the effectiveness of HCQ
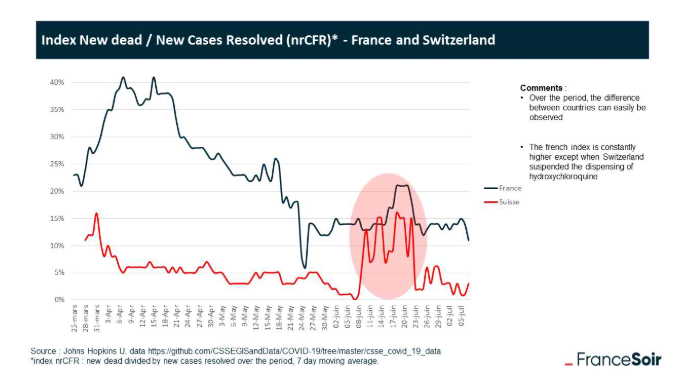
As pointed out by the French site, FranceSoir, with experts on both sides to vindicate their position and invalidate the other, what was needed was clear cut proof that hydroxychloroquine works. Morbidity and mortality data from the period during which Switzerland and France temporarily stopped using it, due to a subsequently retracted Lancet study, provided the necessary proof.
According to the critics of hydroxychloroquine, these 15 days of prohibition should have had no impact on patient survival, but this is not the case: it is enough to look at the evolution over time of the proportion of deaths among newly resolved cases, only to find that hydroxychloroquine, the only molecule banned within this time, works.
Hydroxychloroquine saves lives.
It was enough the collaboration of three internet users to solve this enigma (see the article “story of a discovery”): the discovery of a strict temporary suspension of the HCQ in Switzerland, the nrCFR * efficiency index of treatments, the observation of a “bump” of ~ 2 weeks in this index for Switzerland, the link with the suspension, and finally the analysis concluding with the statistical significance of this correlation with a very high degree of certainty ( > 99%).
This graph shows how the rates of death shot up for both Switzerland and France when HCQ was temporarily banned following the Lancet study and then went down again once its use was reinstated23.
♦ What Global Studies Show
This continuously updated website shows the results of studies investigating the effectiveness of Hydroxychloroquine and other remedies for Covid-1924

Shouldn’t this have been proof enough?
Hydroxychloroquine studies that showed it didn’t work and was even dangerous
Considering the numerous positive studies and physicians’ experiences with hydroxychloroquine, how is it that other studies have reported negative or neutral results for using it as a treatment for Covid-19?
Here are a few of them.
♦ Lancet study – Retracted
A study which was published in the Lancet in late May claimed that not only was there no benefit to hospitalized Covid-19 patients when HCQ was used alone or with a macrolide, but that it decreased in hospital survival and caused fatal heart problems when used for treatment of COVID-1925.
On the basis of this study the WHO stopped the HCQ arm of their Covid therapy trials and the drug was banned in several countries. Just 13 weeks later a retraction was requested by three of the authors. A company named Surgisphere had provided the data purported to come from multiple hospitals in many different countries; the fourth co-author, Sapan Desai, was the company’s chief executive. Questions quickly arose about the data and methodology used and the three coauthors not affiliated with the company attempted to have an independent audit of the data but Surgisphere would not provide the data used for the studies26. 120 medical researchers and professionals wrote to the Lancet questioning the data27.
Additional reference: Todaro, James, M., MD. “A study out of thin air”. May 29, 2020, Medicineuncensored.Com, 2020, https://www.medicineuncensored.com/a-study-out-of-thin-air
Why did the WHO and others accept this study without question when HCQ had over 6 decades of safe use?
♦ WHO Solidarity Trial
The Who Solidarity Trial was conducted with hospitalized patients in many countries. Several different drugs, including hydroxychloroquine, were tested against standard of care. The HCQ arm was initially stopped after the Lancet study (noted above) was published and resumed after it was retracted. However, on June 17 the HCQ arm was stopped permanently.
The WHO’s reported reason for discontinuing the HCQ arm: 
The trial’s Executive Group and principal investigators made the decision based on evidence from the Solidarity trial, UK’s Recovery trial and a Cochrane review of other evidence on hydroxychloroquine.
Data from Solidarity (including the French Discovery trial data) and the recently announced results from the UK’s Recovery trial both showed that hydroxychloroquine does not result in the reduction of mortality of hospitalised COVID-19 patients, when compared with standard of care28.
The WHO made no claim as to the effectiveness of hydroxychloroquine for prophylactic use or to treat patients in a non-hospital setting (see image to right)29 .
Why did the WHO get such different results than other doctors and studies?
Officials in India’s Union Health Ministry and the ICMR (Indian Council of Medical Research) which oversees the country’s successful HCQ protocol, wrote to the WHO advising them of the excessive doses they were using30.
The Indians weren’t the only ones who noticed the problem with the dosing. Dr. Meryl Nass also wrote to the WHO letting them know that they were using lethal doses of hydroxychloroquine.
Why would the WHO authorize trials using doses known to be dangerously excessive?
♦ UK Recovery Trial
The results of the hydroxychloroquine arm of this trial also showed no beneficial results for hospitalized patients31.
The patients in the Recovery Trial were also given the same fatal doses of hydroxychloroquine. The hospitalized patients were also sicker than recommended for use of hydroxychloroquine, which is most effective at the onset of the infection. Some of the patients were so ill that they were not able to even give consent to be part of the trial, in which case their next of kin or even the clinician was permitted to give consent32.
See Dr. Meryl Nass’s review of the studies: “WHO “Solidarity” And UK “Recovery” Clinical Trials Of Hydroxychloroquine Using Potentially Fatal Doses”. 33
How could they not know that the doses they were using were toxic?
♦ Remap-Cap
This trial was also admitting only patients who were already in intensive care units and using the same lethal doses of hydroxychloroquine as the Solidarity and Recovery trials34’35.
♦ Veterans Study
This was a nationwide retrospective study of patients hospitalized with COVID-19 in the US Veterans Health Administration. The investigators found that:
- Hydroxychloroquine (HC) use did not reduce the risk of ventilation or death
- HC with azithromycin (AZ) did not reduce the risk of ventilation or death
- HC, with or without AZ, was associated with longer length of hospital stay36
Didier Raoult and two of his colleagues responded to this study, citing their incredulousness at seeing the results. They had the following issues with the study:
- The group receiving HCQ had twice the amount of Lymphopenia, an illness associated with a high fatality rate, than those who did not receive HCQ.
- The severity of disease was not recorded in either of two tables – one which showed drugs being given before intubation and one where they were given after intubation. Raoult expects that the patients given HCQ following intubation were too sick at that point to receive benefit from the medication.
- 30% of the untreated group actually received azythromycin which is also helpful in Covid-19.
Moreover, incomprehensibly, the “untreated” group actually received azithromycin in 30% of cases, without this group being analyzed in any distinct way. Azithromycin is also a proposed treatment for COVID (Gautret, 2020) with in vitro efficacy (Andreani, 2020), and to mix it with patients who are supposedly untreated is something that is closer to scientific fraud than reasonable analysis37.
♦ French Study
This study assessed the effectiveness of hydroxychloroquine on hospitalized Covid-19 patients who had pneumonia and needed oxygen, but not intensive care, between March 12 and 31, 2020. They concluded that
Hydroxychloroquine has received worldwide attention as a potential treatment for covid-19 because of positive results from small studies. However, the results of this study do not support its use in patients admitted to hospital with covid-19 who require oxygen38.
If these are experts, shouldn’t they have know that hydroxychloroquine is most effective as soon as the virus is detected?
Hydroxychloroquine politicized
Many drugs have been recalled after years of use, even though the manufacturers and FDA had been aware that they were responsible for serious harm and even death. Yet, none of them have been the political football that hydroxychloroquine has become. How could medical experts and agencies allow this to happen, and even be complicit, when we are in the midst of a global pandemic? Would we even have a pandemic if hydroxychloroquine use was recommended39?
♦ President Trump and Hydroxychloroquine
On March 21, 2020, President Trump called HCQ and azythromycin (Zithromax) a game changer in the fight against Covid-19 and asking the FDA to make it readily available40. He also took HCQ prophylactically.
Since then, and on the heels of discredited trials designed to show that HCQ does not work and is even dangerous, headlines have politicized HCQ as the ineffective drug that Trump supports. The articles are then used as an opportunity to malign both Trump and hydroxychloroquine. A few headlines:
Trump touts hydroxychloroquine as a cure for Covid-19. Don’t believe the hype
The anti-malaria drug could be ‘one of the biggest game-changers’, Trump claimed, but there is no magic cure41CDC website drops guidance, anecdotal data on Trump-backed hydroxychloroquine as COVID-19 treatment42
The real reason Trump is obsessed with hydroxychloroquine43
Trump says he takes hydroxychloroquine to prevent coronavirus infection even though it’s an unproven treatment44
Is hatred of the president a reason to deny anyone life-saving medication?
♦ The FDA and hydroxychloroquine
The FDA gave Emergency Use Authorization for HCQ to treat hospitalized Covid-19 patients on March 28, 2020, and rescinded it on June 15, 2020. Here’s what they said (emphasis added):
Today, the U.S. Food and Drug Administration (FDA) revoked the emergency use authorization (EUA) that allowed for chloroquine phosphate and hydroxychloroquine sulfate donated to the Strategic National Stockpile to be used to treat certain hospitalized patients with COVID-19 when a clinical trial was unavailable, or participation in a clinical trial was not feasible. The agency determined that the legal criteria for issuing an EUA are no longer met. Based on its ongoing analysis of the EUA and emerging scientific data, the FDA determined that chloroquine and hydroxychloroquine are unlikely to be effective in treating COVID-19 for the authorized uses in the EUA. Additionally, in light of ongoing serious cardiac adverse events and other potential serious side effects, the known and potential benefits of chloroquine and hydroxychloroquine no longer outweigh the known and potential risks for the authorized use.
…
Chloroquine and hydroxychloroquine are both FDA-approved to treat or prevent malaria. Hydroxychloroquine is also approved to treat autoimmune conditions such as chronic discoid lupus erythematosus, systemic lupus erythematosus in adults, and rheumatoid arthritis. Both drugs have been prescribed for years to help patients with these debilitating, or even deadly, diseases, and FDA has determined that these drugs are safe and effective when used for these diseases in accordance with their FDA-approved labeling. Of note, FDA approved products may be prescribed by physicians for off-label uses if they determine it is appropriate for treating their patients, including during COVID45.
The FDA’s explanation for removing EUA status from HCQ begs the questions:
- Why does the FDA ignore the studies and clinical evidence showing that hydroxychloroquine works for Covid-19 and only attribute dangerous side effects to it from studies they know are problematic, to say the least?
- Why does the FDA claim that HCQ is only dangerous for hospitalized Covid-19 patients while saying that it’s safe and effective for patients with a variety of other illnesses?
The FDA, in their document of questions and answers about the revocation of hydroxychloroquine for EUA stated that “[t]he suggested dosing regimens for CQ and HCQ as detailed in the Fact Sheets are unlikely to produce an antiviral effect46.” This is rather surprising since their recommended dosages were similar to the ones other doctors used successfully to treat their patients, even as they also stated that the proper dosage is unknown. Why did the FDA use the terminology “unlikely to produce” which means that it’s only a supposition that it would not work, not an evidence based conclusion. Did the FDA revoke EUA status based on supposition?
The FDA also included this in their questions and answers:
Q. Will the revocation of the EUA impact the use of hydroxychloroquine sulfate (HCQ) or chloroquine phosphate (CQ) for their FDA-approved uses?
A. … If you are taking HCQ or CQ for their currently approved uses and are diagnosed with COVID-19, use of these drugs may decrease the effectiveness of other potential COVID-19 treatments, specifically, remdesivir. You should discuss options and specific situation with your health care provider47.
Given the evidence that exists for HCQ’s antiviral properties, why did the FDA suggest that patients currently taking HCQ for other illnesses would need a different drug, such as Remdesivir, if they contracted Covid-19?
♦ Dr. Fauci and hydroxychloroquine
In April, 2013, shortly after a new and deadly coronavirus emerged in the Middle East (and before it had been named MERS) there were two already approved antiviral drugs that showed promise in combating it. The drugs, ribavirin and interferon-alpha 2b, used to treat Hepatitis C, were found to stop the new coronavirus from reproducing in lab-grown cells.
Even though they had only shown to be effective in petri dishes, Tony Fauci, director of the infectious-disease institute which financed the research on those drugs was encouraged. He said (emphasis added):
We don’t have to start designing new drugs,” a process that takes years, Fauci says. “The next time someone comes into an emergency room in Qatar or Saudi Arabia, you would have drugs that are readily available. And at least you would have some data.”
Even though the treatment hasn’t gone through definitive trials, Fauci says, “if I were a physician in a hospital and someone were dying, rather than do nothing, you can see if these work48.
Chloroquine and Hydroxychloroquine were not only proven effective against coronavirus SARS Cov-1, in cells in a petri dish in 2005, they were also used successfully by doctors to help their patients fight the Covid-19 coronavirus, SARS Cov-2, which shares 70% of the genome of SARS Cov-1. Yet, Dr. Fauci, called evidence that HCQ works for Covid-19 patients “anecdotal”49.
Trump used the same argument that Fauci made for ribavirin and interferon-alpha 2b to treat MERS – the essence of time. However, what was evidence enough for Fauci in 2013, was not evidence enough for him in 2020:
… Trump said America doesn’t have time “to take a couple years” to test the efficacy of the drug in treating Covid-19.
But Dr Anthony Fauci, the nation’s top doctor on infectious diseases and a key member of the White House taskforce, was adamant there was nothing to suggest the medicine had any benefit against coronavirus.
“In terms of science, I don’t think we can definitively say it works,” he told CBS’s Face the Nation.
“The data are really just at best suggestive. There have been cases that show there may be an effect and there are others to show there’s no effect.”
…
On 21 March, the day after another nationally televised Trump claim that the drug “looked promising”, Fauci was asked directly if it could be used to treat Covid-19.
“The answer is no,” he said50.
Fauci was also very critical of the Henry Ford study showing that HCQ works. He said:
… The fact is it is not a randomized placebo-controlled trial. The point that I think is important, because we all want to keep an open mind, any and all of the randomized placebo-controlled trials, which is the gold standard of determining if something is effective, none of them have shown any efficacy for hydroxychloroquine51.
Responding to Fauci’s criticism of the Ford Study, the Chief Academic and Chief Clinical Officers noted that while the trials Fauci is looking for are the gold standard, they take years to complete and therefore “a whole scientific field exists in which scientists examine how a drug is working in the real world to get as best an answer as they can as soon as possible”.
They also lamented that “the political climate that has persisted has made any objective discussion about this drug impossible”52.
Tom Frieden, former CDC director, in a review of the different types of evidence for making health decisions, showed that the randomized placebo-controlled trial was not always the best, particularly in emergency situations. He explained that many other types of evidence were equally valid, depending on the circumstances. Case reports and series, (such as those of doctors who’ve used HCQ to treat their Covid-19 patients) he considered good to identify treatments for illnesses with poor prognosis.53.
What could have caused Fauci, the head of the Coronavirus Task Force and long-time director of the NIAID, to employ a double standard when it came to Covid-19 patients and hydroxychloroquine, especially during a pandemic when it has the potential to save lives, and slow down or even halt spread of the disease?
♦ The pharmaceutical industry, government, and hydroxychloroquine
In 2013, out of concern for people becoming very ill and dying from a new coronavirus, Fauci agreed that using established drugs merely proven effective in a petri dish was acceptable, but in 2020, just 7 years later nothing but a randomized placebo-controlled trial was good enough to approve a drug for this year’s pandemic coronavirus, Covid-19, despite the fact that many doctors had been using hydroxychloroqouine successfully. These doctors were seeing patients every day; Fauci had not seen a patient in many years.
At the same time that Fauci was discrediting hydroxychloroquine, he was involved in trials for, and touting, another drug to treat patients with Covid-19, Remdesivir, developed by Gilead Sciences. This drug trial was sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, for which Fauci works54. The federal government had provided a lot of funding toward its development over the past years, as a drug to fight coronaviruses. Remdesivir was tested at high-risk security labs run by the federal government55.
In May, Remdesivir received EUA status for moderately ill hospitalized patients, in June EUA status was removed from hydroxychloroquine, and in August, Remdesivir was given Emergency Use Authorization for all hospitalized patients56’57. Gilead planned to charge over $3,000 per course of treatment. Noting that there are no FDA approved drugs to fight the coronavirus, remdesivir was presented as
… the first antiviral medication to show effectiveness against the novel coronavirus in human clinical trials in limiting hospitalization stays58.
Off-patent hydroxychloroquine with zinc, and zythromax cost only a few dollars per course of treatment.
Could this be the reason that the FDA panned hydroxychloroquine and removed its EUA status for hospitalized patients?
France also discontinue approval of HCQ for Covid-19. Professor Raoult testified before the French National Assembly that he had received death threats shortly after recommending HCQ for Covid patients. A police investigation found that the threats were made by the scientist in Nante University who had received the most grant money from Gilead Science 59.
Regarding the controversy over HCQ in France, an article in the Journal of Sociology had this to say:
… It centres on the therapeutic proposal of a Marseilles doctor, who has become a very divisive ‘star’ in public debates over the efficacy of treatment. The author shows that competition between this doctor’s proposal and the commercial hopes of a major pharmaceutical company plays an important role. This company has managed to create links of interest with many other major doctors, some of whom are at the heart of the decision-making process concerning the management of the health crisis …60.
Gilead Sciences may have even involved in the retracted Lancet study which purported to show that HCQ doesn’t work. The study received funding from the William Harvey Distinguished Chair in Advanced Cardiovascular Medicine at Brigham and Women’s Hospital; the chair is held by Dr. Mandeep Mehra, one of the authors of the study. Brigham Health has a major contract with Gilead Sciences related to the development of Remdesivir as a treatment for Covid-1961.
Perhaps more important than Remdesivir is the hoped for vaccine for Covid-19, expected to being given to every human being on the planet. If those plans come to fruition, 7.8 billion people will be vaccinated; this represents an enormous amount of profit. Not only that, but similar to the way the US government has been involved in the development of Remdesivir, the US Dept. of Health & Human services has provided close to $1 billion to Moderna to develop their mRNA vaccine against this coronavirus and to help scale up production for it, as well. This means that the cost of developing the vaccine was not born by the company, but by the American public.62’63. In addition, they have received millions of dollars in awards from the US military Defense Advanced Research Projects Agency in patent applications the company filed for vaccines. It is highly likely that other government funding the company received over the years led to the technology being used for the vaccine for Covid-19. In addition, the National Institutes of Health may own mRNA-1273, Moderna’s Covid-19 vaccine candidate64.
See also:
Dr Fauci says he hopes we don’t have herd immunity, pushes for vaccines65
Letter to Dr. Fauci, subject: Your Official Response to SARS-CoV-2 / COVID-19 Related Questions66.
Patents by Inventor Anthony S. Fauci 67
Bobby Kennedy Jr. Claims Dr. Fauci and Gates Foundation Will Make Billions on Coronavirus Vaccine68
According to the CDC, government employees who are listed on patents receive royalties of up to $150,000 per year69.
‘Bad Optics’ Or Something More? Moderna Executives’ Stock Sales Raise Concerns70
In quest for vaccine, US makes ‘big bet’ on company with unproven technology71
Is corruption and conflict of interest in the public’s best interest? Does this serve the greater good?
Social media and hydroxychloroquine
Midst spurious claims of misinformation and violation of community standards, social media giants YouTube, Twitter, Facebook, Instagram, and Google, have been increasingly censoring the information the public is permitted, in their opinion, to know about. While the media has been practicing viewpoint censorship for a number of years already, it has been ramped up since the pandemic was announced and hydroxychloroquine became politicized. Online censorship is the modern day form of book burning as videos and even entire pages, channels, and accounts that have been online for many years, have been removed without any warning or ability for the owners to contest the decision.
Facebook, Twitter and YouTube pull ‘false’ coronavirus video after it goes viral72.
YouTube, Facebook split on removal of doctors’ viral coronavirus videos73
Google Removed Key Papers and Videos on Hydroxychloroquine74
The Media Sabotage of Hydroxychloroquine Use for COVID-19: Doctors Worldwide Protest the Disaster75
YouTube removing content that goes ‘against World Health Organization recommendations,’ CEO says76
America’s Frontline Doctors’ May Be Real Doctors, But Experts Say They Don’t Know What They’re Talking About77.
Is this the role of social media? Should we allow tech giants to deny US citizens their constitutional right to free speech or the ability to make their own informed opinions?
About our medical experts
Fauci claims that there is an anti-science bias in America and that science is truth. However,
Manipulation of data, trial studies designed to kill, double standards applied for approval of drugs in emergency situations, threats against those with different answers … is neither science nor truth; it’s fraud and it’s criminal. Pushing back against this does not make one anti-science or the US a country with an anti-science bias.
Instead of the truth, we have been given a troubling view of the local, national, and global health authorities that we have been looking up to all these years. 78
If this is the behavior of experts and authorities during a global pandemic, can we trust them at all, about anything? Who are they really serving?
Scientific Consensus
The scientific process is one of testing hypothesis and refining judgments. Science is never served by stifling the voice of an opposing viewpoint.
Astrophysicist Ethan Siegel contends that laypeople cannot possibly understand science.
In an article in Forbes magazine, “You Must Not ‘Do Your Own Research’ When It Comes To Science”, Siegel exhorts individuals not to do their own research, but leave it up to the “experts”. Since it is not their field of expertise, he claims that lay people are more likely than not to make dangerous, even life-threatening errors. Accordingly:
When laypersons espouse opinions on those matters, it’s immediately clear to us where the gaps in their understanding are and where they’ve misled themselves in their reasoning. When they take up the arguments of a contrarian scientist, we recognize what they’re overlooking, misinterpreting, or omitting. Unless we start valuing the actual expertise that legitimate experts have spent lifetimes developing, “doing our own research” could lead to immeasurable, unnecessary suffering.
We must never listen to that contrarian scientist. We want consensus, he says.
We think that, just by applying our brainpower and our critical reasoning skills, we can discern whose expert opinions are trustworthy and responsible. We think that we can see through who’s a charlatan and a fraud, and we can tell what’s safe and effective from what’s dangerous and ineffective.
Except, for almost all of us, we can’t. Even those of us with excellent critical thinking skills and lots of experience trying to dig up the truth behind a variety of claims are lacking one important asset: the scientific expertise necessary to understand any finds or claims in the context of the full state of knowledge of your field. It’s part of why scientific consensus is so remarkably valuable: it only exists when the overwhelming majority of qualified professionals all hold the same consistent professional opinion. It truly is one of the most important and valuable types of expertise that humanity has ever developed79.
- Why would any scientist tell people not to try to understand and educate themselves about some of the matters most relevant to their health and well-being?
- Why would any scientist tell people that they are not smart enough to do that?
But let’s consider the validity of scientific consensus. Can it be that the contrarian scientist is always wrong and that all we need to know is what most scientists agree to?
Here is science fiction novelist Michael Crichton’s view on science and consensus:
Let’s be clear: the work of science has nothing whatever to do with consensus. Consensus is the business of politics. Science, on the contrary, requires only one investigator who happens to be right, which means that he or she has results that are verifiable by reference to the real world. In science consensus is irrelevant. What is relevant is reproducible results. The greatest scientists in history are great precisely because they broke with the consensus. There is no such thing as consensus science. If it’s consensus, it isn’t science. If it’s science, it isn’t consensus. Period.
…
… Finally, I would remind you to notice where the claim of consensus is invoked. Consensus is invoked only in situations where the science is not solid enough …
As far as lone scientists, the contrarian ones, can be correct, even if it takes more than a hundred years to acknowledge it. He brings us several examples:
- In 1795, Alexander Gordon of Aberdeen suggested that the fevers were infectious processes, and he was able to cure them. The consensus said no.
- In 1843, Oliver Wendell Holmes claimed puerperal fever was contagious, and presented compelling evidence. The consensus said no.
- In 1849, Semmelweiss demonstrated that sanitary techniques virtually eliminated puerperal fever in hospitals under his management. The consensus said he was a Jew, ignored him, and dismissed him from his post.
- In the early 19oos, Joseph Goldberger determined that pellagra was due to diet, not germs. It was not until the 1920s that his findings were finally accepted80.
British economist, John Kay, says this about science and consensus:
… Consensus is a political concept, not a scientific one.
Consensus finds a way through conflicting opinions and interests. Consensus is achieved when the outcome of discussion leaves everyone feeling they have been given enough of what they want. The processes of proper science could hardly be more different. The accomplished politician is a negotiator, a conciliator, finding agreement where none seemed to exist. The accomplished scientist is an original, an extremist, disrupting established patterns of thought. Good science involves perpetual, open debate, in which every objection is aired and dissents are sharpened and clarified, not smoothed over.
Often the argument will continue for ever, and should, because the objective of science is not agreement on a course of action, but the pursuit of truth. Occasionally that pursuit seems to have been successful and the matter is resolved, not by consensus, but by the exhaustion of opposition81.
- Is there solid science for denying the use of hydroxychloroquine for Covid-19?
- Have we exhausted all opposition?
- If consensus is a political construct and has no place in science, why are we being told otherwise?
- Who gains and who loses when scientists deny the validity of divergent conclusions?
In conclusion
If there are rats in the cellar you are most likely to see them if you go in very suddenly.
But the suddenness doesn’t create the rats: it only prevents them from hiding82.
The highly visible controversy, confusion, and politicization regarding the use of hydroxychloroquine for Covid-19 may make it seem like this is an unusual situation. The truth, however, is that this has been the reality for decades83’84. The suddenness of the Covid-19 pandemic and the goldmine that an expensive patented cure (and ultimately, global vaccine campaign) could be for those involved in its development and sale, has merely brought to light what those who we presumed to be trustworthy experts have been doing in the dark all along.[/ref].
Footnotes



