Adverse Effects You Should Know About
The whooping cough vaccine, in addition to its waning immunity and failure to stop transmission, as discussed in our previous post “Does the Whooping Cough Vaccine Work?“, has many serious adverse effects. Physicians for Informed Consent’s (PIC) Vaccine Risk Statement details them:
Common side effects of the DTaP vaccine include fever, loss of appetite, vomiting, and fatigue/weakness. A more serious potential side effect is seizure, which may occur in about 1 in 683 children vaccinated with DTaP. Although severe side effects have been observed following DTaP vaccination, including neurological disorders (e.g., encephalitis, infantile spasms, ataxia, autism, transverse myelitis, optic neuritis, multiple sclerosis, Guillain-Barré syndrome, and Bell’s palsy), autoimmune diseases (e.g., chronic urticaria, serum sickness, and arthropathy), myocarditis, and sudden infant death syndrome, the Institute of Medicine (IOM) states that “the evidence is inadequate to accept or reject a causal relationship between diphtheria toxoid-, tetanus toxoid-, or acellular pertussis-containing vaccine” and those conditions.
Neurotoxic Aluminum in the Vaccines
PIC also includes the high levels of aluminum in the vaccine as a risk.
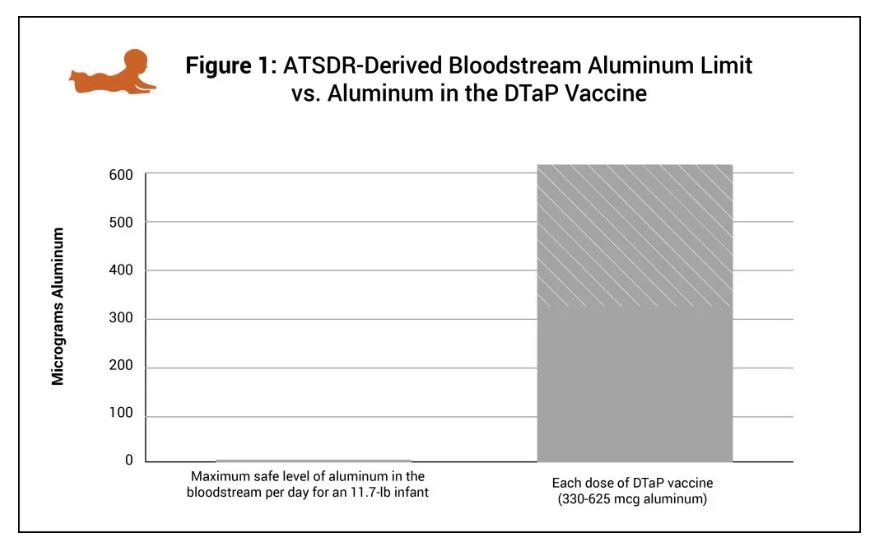
The DTaP vaccine contains 330 mcg to 625 mcg of aluminum, an amount 60 to 120 times greater than the maximum safe level of aluminum in the bloodstream per day for an 11.7-pound (5.3-kilogram) infant, derived from the Agency for Toxic Substances and Disease Registry, a division of the U.S. Department of Health and Human Services (HHS) (Fig. 1).
PIC provided the following graph showing the amount of allowable aluminum in the bloodstream for an 11.7 lb. infant vs the amount that is in one dose of the DTaP vaccine.
Aluminum is used in many vaccines as an adjuvant to provoke an immune response. However, as Rodef Shalom has written, here and here, it is a known toxin that has no purpose in the human body and is associated with autism.
Potential harms they don’t test for
Rare and long-term injury
The DTaP clinical trials are too small and not conducted for long enough to identify unusual adverse events or those that take years to appear. PIC quoted the CDC, stating:
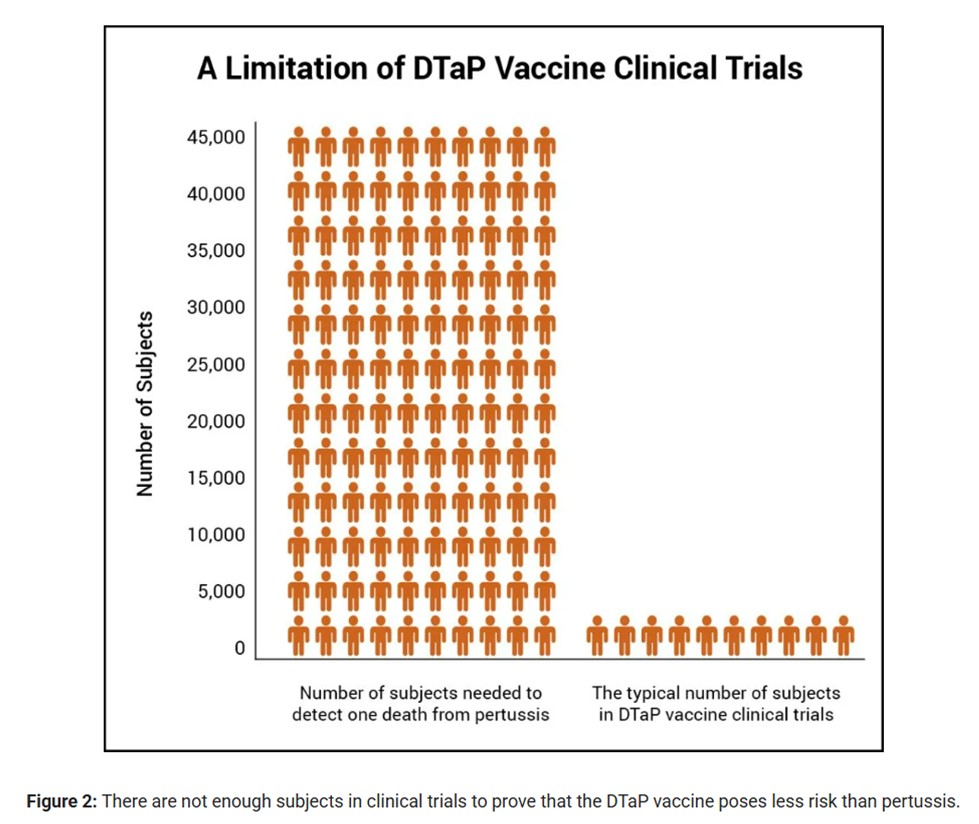
Prelicensure trials are relatively small — usually limited to a few thousand subjects — and usually last no longer than a few years… Prelicensure trials usually do not have the ability to detect rare adverse events or adverse events with delayed onset.
The trials are also too small to determine if the vaccine causes permanent injury or death. PIC notes that the risk of death for infants under one year without mass vaccination is 1 in 46,000. This means that “a few thousand subjects in clinical trials are not enough to prove that the DTaP vaccine causes less permanent injury or death than pertussis.” However, as their graph (below) shows, the trials have only a few thousand subjects compared to the many thousands that would be needed to detect such harms.
Cancer, genetic mutations, and infertility
Like most vaccines, the pertussis vaccines have not been tested for the most serious of potential adverse effects – causing cancer, genetic mutations, or impairing the ability to have children. This information is found in Section 13.1 of vaccine package inserts. The following are screenshots of Section 13.1 for the Infranrix and Daptacel vaccines, the two given to newborns and young children.
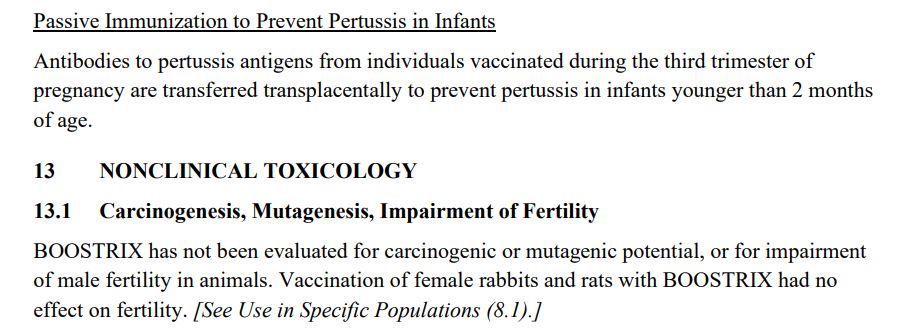
Below is the screenshot for Section 13.1 of the vaccine package insert for the TDaP vaccine, Boostrix, recommended for individuals 10 years and above, including pregnant women. It states that it was not tested in animals for its ability to cause cancer or genetic mutations. It was only tested on female animals, not males, for impairment of fertility.
In its section Vaccination During Pregnancy (p15), we learn that there was a clinical trial that included pregnant women. Below is the screenshot of the section.
It is impossible to tell whether human fertility was affected since the insert states that the number of women who reported a pregnancy was far greater than the number of pregnancies with known outcomes.
According to the insert, human data (p17) from the U.S. pregnancy exposure registry indicated that women who received the vaccine during the trial were followed for 17 years with no major birth defects reported. However, of the 1523 prospective reports of pregnancy, only 256 had known outcomes. Additionally, there was post-marketing data and U.S. spontaneous reports for 810 “prospective reports of exposure to BOOSTRIX during pregnancy,” yet, they only had 138 known pregnancy outcomes.
This means that they do not know the actual degree of birth defects or any other problems that may have arisen in the rest of the women or their fetuses or infants for whom they have no further information. Additionally, “major birth defects” is an undefined and uncertain term and may not be the only harm that can be caused to the woman or her baby.
Non-pregnant trial participants (p6) were only followed for up to 6 months.
PIC asks: “Is the DTaP Vaccine Safer than Pertussis?” And the answer is, we don’t know. They state:
Because permanent sequelae (aftereffects) from pertussis are so rare, the level of accuracy of the research studies available is insufficient to prove that the DTaP vaccine causes less permanent injury or death than pertussis.
As for the TDaP vaccine, they note that it is just a different formulation of the DTaP vaccine; all clinical trials, epidemiological studies, and surveillance systems are similar to those of the DTaP vaccines. Therefore, we cannot know if the TDaP vaccine is safer than pertussis either.
Feature image by Mart Production