Compilation of Section 13 of various vaccine package inserts
How Vaccines Can Cause Childhood Cancers
What health organizations say about childhood cancers
American Cancer Society
“Cancers can be caused by DNA changes that keep oncogenes turned on, or that turn off tumor suppressor genes …
“Some children inherit DNA changes (mutations) from a parent that increase their risk of certain types of cancer. …
“But most childhood cancers are not caused by inherited DNA changes. …
“Acquired mutations are only in the person’s cancer cells and will not be passed on to his or her children. … “
“But the causes of DNA changes in most childhood cancers are not known1.”
The National Cancer Institute:
“A number of studies are examining suspected or possible risk factors for childhood cancers, including early-life exposures to infectious agents; parental, fetal, or childhood exposures to environmental toxins such as pesticides, solvents, or other household chemicals; parental occupational exposures to radiation or chemicals; parental medical conditions during pregnancy or before conception; maternal diet during pregnancy; early postnatal feeding patterns and diet; and maternal reproductive history. Researchers are also studying the risks associated with maternal exposures to oral contraceptives, fertility drugs, and other medications; familial and genetic susceptibility; and risk associated with exposure to the human immunodeficiency virus (HIV)2.”
What about vaccines?
Vaccines include many of the cancer risk factors cited above:
- Carcinogenic chemicals
- Foreign fragmented DNA which can cause DNA mutations and are tumorigenic.
- Infectious agents – Known and unknown viruses and retroviruses which can turn off tumor suppressor genes and which can be cancer causing.
Among the toxins in vaccines associated with cancers are:
Formaldehyde:
Formaldehyde is a known human carcinogen. It has been associated with several different types of cancers, particularly cancers of the hematopoietic and lymphatic systems, nasopharyngeal cancer, and lung cancer. Tests done on formaldehyde toxicity have focused on inhalation, drinking, and contact with skin; no tests have been performed to determine the toxicity of injected formaldehyde. It is a known DNA adduct, a chemical that bonds to a piece of DNA. This is a process which could be the start of a cancerous cell since when a chemical binds to DNA, the DNA gets damaged, resulting in abnormal replication3.
25 of the 53 vaccines listed by the CDC have formaldehyde in them, including DTaP, Hib, HPV, Hep A, Hep B, Influenza, Meningitis, and Polio.
beta-Propiolactone:
“… No information is available on the chronic (long-term), reproductive, developmental, or carcinogenic effects of beta-propiolactone in humans. Squamous cell carcinomas of the forestomach have been reported in orally exposed rats. In dermally exposed rodents, skin tumors have been observed4.”
Foreign DNA – Aborted fetal stem cell DNA:
Several live virus vaccines are cultured on human embryonic stem cell lines taken from aborted human fetuses in the 1960s. They are identified as MRC-5 and WI-38; the DNA in the aborted fetal cell lines had been fragmented under the supposition that injecting whole DNA is dangerous, but fragments are not5.
Insertional mutagenesis:
Through a process known as insertional mutagenesis, exogenous DNA can be incorporated into the cells of a host and cause carcinogenic mutations. Human embryonic stem cells are known to be tumorigenic6.
Insertional mutagenesis, called transcension at the time, was known since the 1960s when scientists Anker and Stroun discovered that exogenous bacterial DNA will be taken up and incorporated into the host DNA of animals. In the 1990s they discovered that free-floating DNA fragments were associated with cancers7 8.
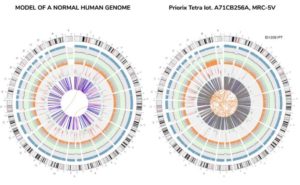
Whole, tumorigenic DNA was recently found in the Priorix Tetra Vaccine (for measles, mumps, rubella, and varicella) by Corvelva, an independent lab. Below is an image of a normal human genome (complete DNA) and the one found in the vaccine9.
Infectious Agents
Retroviruses:
“Integration of new DNA into a cellular chromosome can alter the activity of nearby genes, sometimes affecting subsequent cell growth. A potent form of insertional mutagenesis involves integration of retroviral DNA produced by reverse transcription, a required step in the replication of retroviruses. In recent years retroviral replication has been adapted to allow new gene addition by retroviral vectors. Early in the history of retrovirus research, analysis of insertional mutagenesis in laboratory animals was found at times to result in transformation, leading to the discovery of cellular proto-oncogenes. In-depth analysis of the genetic consequences showed that integration of retroviral DNA could alter the gene activity in a variety of ways10.”
“Insertional mutagenesis is a major concern with all of the integrating viral vectors and has been subjected to intense scrutiny since four cases of T-cell leukemia occurred in human pediatric patients with X-SCID 31–68 months after they received γ-retroviral vector-modified autologous HSC. This concern was further heightened when linker-mediated PCR analysis of lymphocytes from these patients revealed that insertional mutagenesis had occurred in all four patients and was at least partially responsible for the observed leukemogenesis11.”
Virus-Chemical Carcinogen Interactions
Studies in the 1960s revealed a synergistic effect between viruses that would normally not form cancers and carcinogenic chemicals that were in too small doses to cause tumors. A 1961 report on a study in mice revealed tumor formation in rats that were simultaneously exposed to injected virus and carcinogens:
“Single doses of pairs of viruses and organic carcinogens (in amounts too small in themselves to induce tumors) were administered to male Swiss mice free of polyoma virus. Malignant tumors developed in groups of mice injected with five of the carcinogen-virus pairs. 12”.
The interaction between viruses and carcinogens was reviewed and confirmed by other studies13.
These studies raise the probability that vaccine viruses when combined with even small amounts of environmental pollutants, pesticides, and other toxic agricultural by products found in food, can induce cancer formation14.
Polio vaccine and cancer
HeLa Cells
In order to produce a vaccine, the virus first needs to be grown on a cellular medium. Salk initially used HeLa cells to grow the poliovirus and test the vaccine before it was manufactured for human use. HeLa cells were cells from the cervical cancer tumor of a women named Helen Lacks 15.
Cancer causing virus in the polio vaccine
In 1960, the Salk killed virus polio vaccine, grown in rhesus monkey kidney cell cultures, was discovered by Bernice Eddy, an NIH (National Institute of Health) scientist, to harbor a cancer causing virus. Later that year it was identified as SV40 (the 40th known simian (monkey) virus) which was shown to cause cancer in rodents. Despite her warnings, the vaccine was not withdrawn from the market and, with full knowledge of the health agencies, was given to more American children. In the US, 100 million children were given this vaccine between 1954 and 1963 when it was discontinued in favor of the live oral polio Sabin vaccine. The Sabin vaccine was grown on African Green Monkey cells since they were said to rarely be affected by SV4016. However, the vaccine seed stocks used for the Sabin vaccine were from the Salk vaccine. Despite the manufacturer’s claims to the contrary, it was discovered that the seed stocks from the Salk vaccine were not adequately tested to make sure that they were cleared of SV40. Potentially SV40-contaminated oral polio vaccine was given to children through 1999, when it was discontinued in the US and replaced by the inactivated vaccine.
“The absence of confirmatory testing of the seeds, as well as testimony of a Lederle manager, indicate that this claim of removal of SV40 and the testing for SV40 in all the seeds cannot be fully substantiated. These legal documents and testimony indicate that the scientific community should not be content with prior assumptions that SV40 could not have been in the oral polio vaccine. Only further investigation by outside scientific and independent researchers who can review the test results claimed in the January 1997 meeting and who can conduct their own independent evaluations by testing all the seeds and individual mono-valent pools will assure that SV40 has not been present in commercially sold oral poliovirus vaccine manufactured by Lederle17.”
Hilleman admits contamination and expects cancers
Maurice Hilleman, chief of Merck’s vaccine division at the time SV40 was found to have contaminated the polio vaccine, tested and found SV40 in the Sabin vaccine that was being put through clinical trials in Russia at the time. It was already known that other vaccines grown on living tissue contained carcinogenic viruses, such as the smallpox vaccine containing the leukemia virus. Hilleman felt that SV40 could have even worse long term cancer effects. There was no press release about these findings. Hilleman said this was a scientific affair within the scientific community. Watch the interview with Dr. Hilleman below as he describes this period.
Increased cancers
An analysis of the SEER government database from 1973-1993 showed greater rates of ependymomas (37%), osteogenic sarcomas (26%), other bone tumors (34%) and mesothelioma (90%) among those who were administered SV40 containing vaccines that those of an unexposed birth cohort18.
In 2001, the Institute of Medicine Immunization Safety Review Committee stated that:
“… in light of the biological evidence supporting the theory that SV40 contamination of polio vaccines could contribute to human cancers, the Committee recommends continued public health attention in the form of policy analysis, communication and targeted biological research19.”
Molecular biology and epidemiologic studies suggest that SV40 may now be passed from person to person by horizontal infection, in absence of a SV40 containing vaccine. SV40 footprints have been found in high prevalence in human brain and bone tumors, mesotheliomas, and lymphomas and with kidney diseases, and at lower prevalence in blood samples from healthy donors20.
When Eddy discovered the tumorigenicity of the vaccine in 1960, her boss at the NIH did not believe her. Her discovery also threatened one of the country’s most important public-health programs and she was removed from her vaccine regulatory duties and her laboratory.
In the 1990s another NIH employee, Michele Carbone, studying the way SV40 induces cancer in animals, tested human mesothelioma tumour biopsies at the National Cancer Institute. He discovered that 60% contained SV40 DNA and in most of them, the monkey virus was active and producing proteins. He published his findings in the May 1994 edition of Oncogene; the NIH refused to publicize them; other employees promptly contested his findings. After leaving the NIH, Carbone discovered that SV40 disables tumour suppressor genes in human mesothelioma. In July 1997, he published his results in Nature Medicine21.
CDC on the polio vaccine and cancer
This page on the relationship between the polio  vaccine and cancer caused by SV40 (image on right), web archived but removed from the CDC website in October 2019), denied that the OPV contained SV4022. However, another CDC webpage acknowledges that the Sabin vaccine also contained SV40: “Most of the contamination was in the inactivated polio [Salk] vaccine (IPV), but it was also found in oral polio [Sabin] vaccine (OPV)23.”
vaccine and cancer caused by SV40 (image on right), web archived but removed from the CDC website in October 2019), denied that the OPV contained SV4022. However, another CDC webpage acknowledges that the Sabin vaccine also contained SV40: “Most of the contamination was in the inactivated polio [Salk] vaccine (IPV), but it was also found in oral polio [Sabin] vaccine (OPV)23.”
To this day, despite a plethora of evidence showing otherwise, the CDC contends that the evidence is not sufficient to show that SV40 causes cancers in people24.
Vaccine strain polio virus shedding
In March 1959, Albert Sabin published a paper in the British Medical Journal, Present Position of Immunization Against Poliomyelitis with Live Virus Vaccines25, where he acknowledged that vaccine strain shedding of the polio virus was a known fact.They were monitoring the situation carefully and testing the feasibility of the oral polio vaccine in trials with hundreds of thousands of children.
Growing vaccines on human tumor cell lines
In 2001 an FDA document, “Designer” Cells as Substrates for the Manufacture of Viral Vaccines, discussed the use of cell lines made tumorigenic in order to grow particular viruses for vaccines which do not grown well on the standard substrates, such as the AIDS virus. Potential dangers of using these vaccines were reviewed, including the ability to cause cancer or infections in the recipient and mitigating factors, contamination by adventitious agents (toxins and viruses that are often infectious) for which assays to detect unknown agents may become available, and the risk of TSE, transmissible spongiform encephalopathy, and possible methods of detection 26 27." target="_blank" rel="noopener noreferrer">https://wayback.archive-it.org/7993/20170404095417/https:/www.fda.gov/ohrms/dockets/ac/01/transcripts/3750t1_01.pdf[/ref].
In 2014 the FDA convened a meeting to discuss the use of tumorigenic cell lines from human tumors in the production of vaccines.
“…three examples of human tumor derived cells that are proposed for use as vaccine cell substrates.vaccine cell substrates. … The A549 lung adenocarcinoma cell line, which is proposed for use for adenovirus-vectored vaccines for antigens like influenza, HIV or anthrax.CEM derived cells, which come from a lymphoblastic T cell leukemia, proposed for use in an inactivated HIV vaccine, and HeLa cells, which comes from cervical carcinoma, proposed for use for an AAV-vectored HIV vaccine.
…
“So the big question that we really have to consider, then, is: Is there a potential risk for making vaccines in tumor derived cells? The theoretical risk comes from the fact that, in contrast to many other products, vaccines are often difficult to purify to high levels. And that then leads to the question of whether residual cellular components from tumor-derived cells could pose a safety concern28.”
Sometime afterwards, the FDA initiated a biologics research project to investigate the dangers of latent cancer causing viruses in tumorigenic cell lines.
“The use of tumorigenic and tumor-derived cells is a major safety concern due to the potential presence of viruses such as retroviruses and oncogenic DNA viruses that could be associated with tumorigenicity. Therefore, detection of persistent, latent DNA viruses, and endogenous retroviruses in vaccine cell substrates is important for vaccine safety, particularly in the development of live viral vaccines, where there are no or minimal virus inactivation and removal steps during vaccine manufacturing29.”
Vaccine safety testing
“Mutagen and carcinogen are two physical, chemical or biological factors that may cause changes in normal cell division in organisms. Approximately, 90% of the carcinogens are mutagens. The somatic cell mutations can cause cancers. The main difference between mutagen and carcinogen is that mutagen causes a heritable change in the genetic information of an organism whereas carcinogen causes or promotes cancer in animals and humans. Mutagenesis is the mechanism by which the change in the genetic material occurs…” 30
Per section 13 of all vaccine package inserts, vaccines have not been tested for carcinogenicity, mutagenicity, or impairment of fertility [in humans]31.
Why not?
Related: Increase in Childhood Cancers
Footnotes